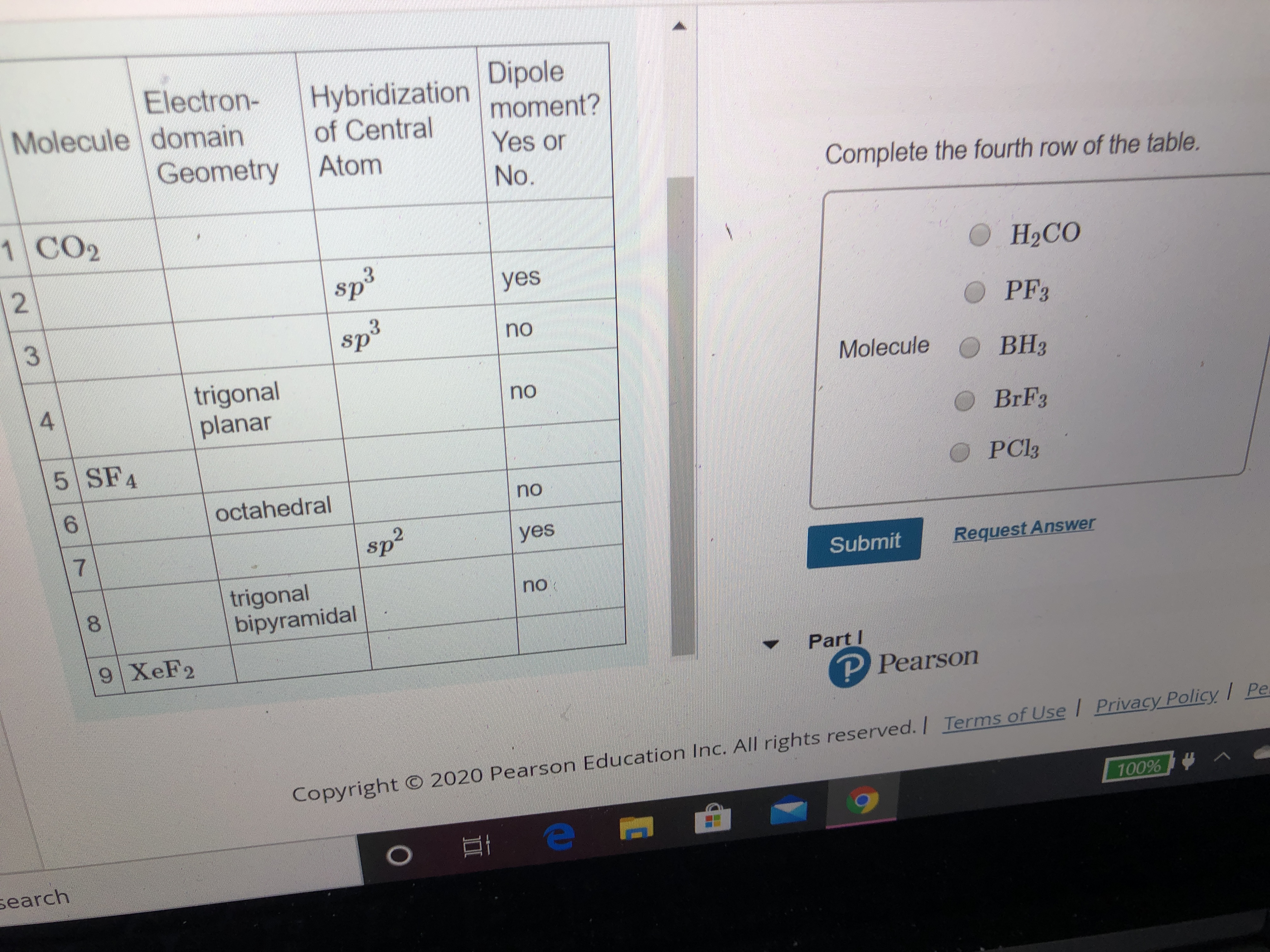
![SOLVED: I need to find the oxidation state, hybridization of the central atom, bond angle, nature of bonding, and color of [Co(NH3)6]Br3. Please describe it. SOLVED: I need to find the oxidation state, hybridization of the central atom, bond angle, nature of bonding, and color of [Co(NH3)6]Br3. Please describe it.](https://cdn.numerade.com/ask_previews/9dd48213-7427-4942-a69b-33d891dfdf69_large.jpg)
SOLVED: I need to find the oxidation state, hybridization of the central atom, bond angle, nature of bonding, and color of [Co(NH3)6]Br3. Please describe it.
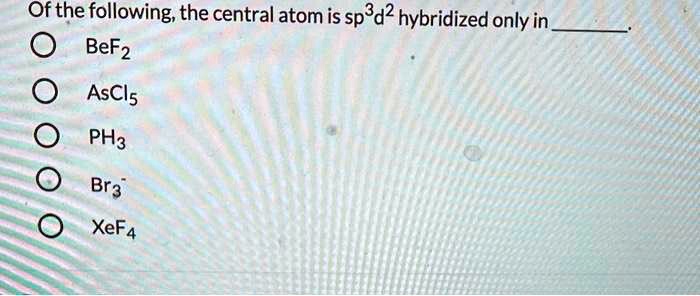
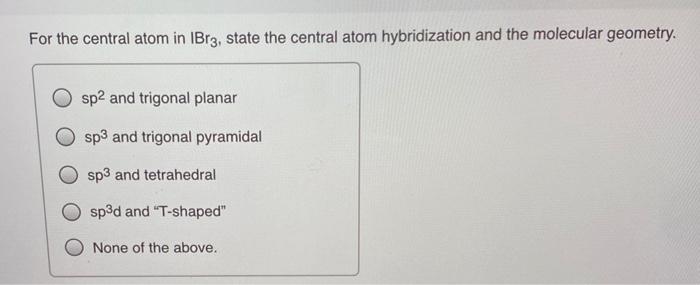
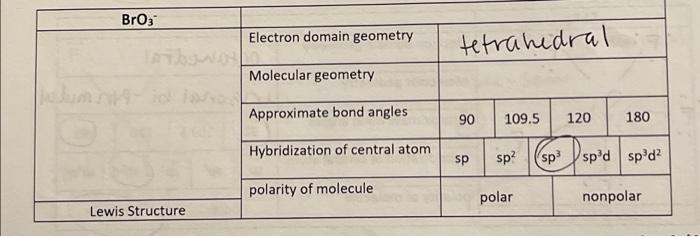
SOLVED: Of the following, the central atom is sp3d2 hybridized only in BeF2, AsCl3, PH3, Br3, O, and XeF4.

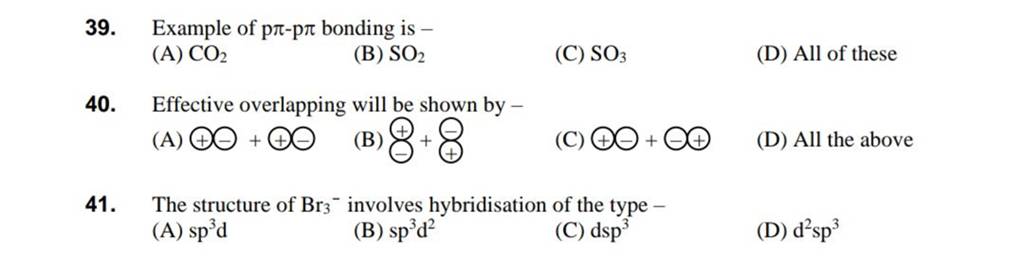
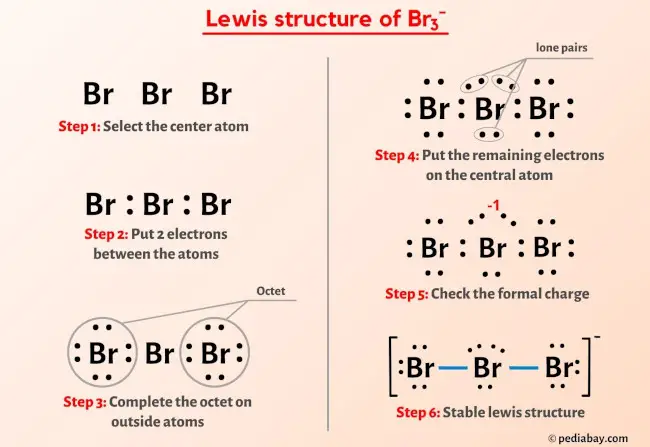
While I3- and Br3- are both stable molecules, F3- is not a stable molecule. Provide an explanation. | Homework.Study.com
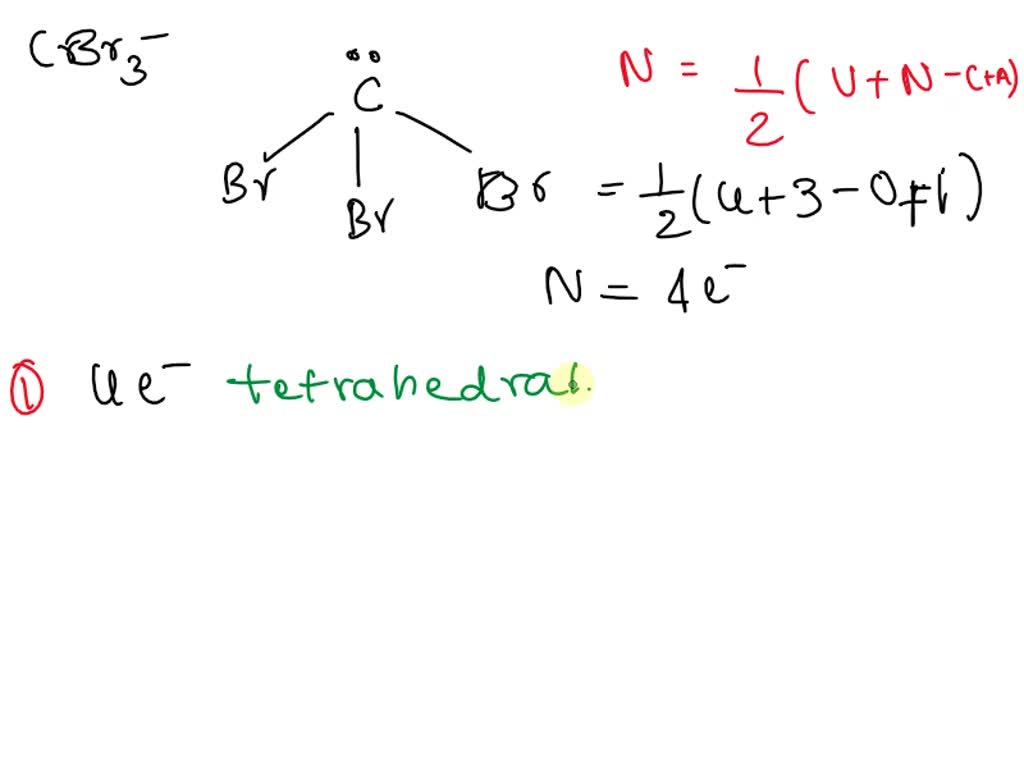
SOLVED: Give the electron geometry, molecular geometry, and hybridization for CBr3-. Electron geometry: trigonal planar Molecular geometry: trigonal pyramidal Hybridization: sp3

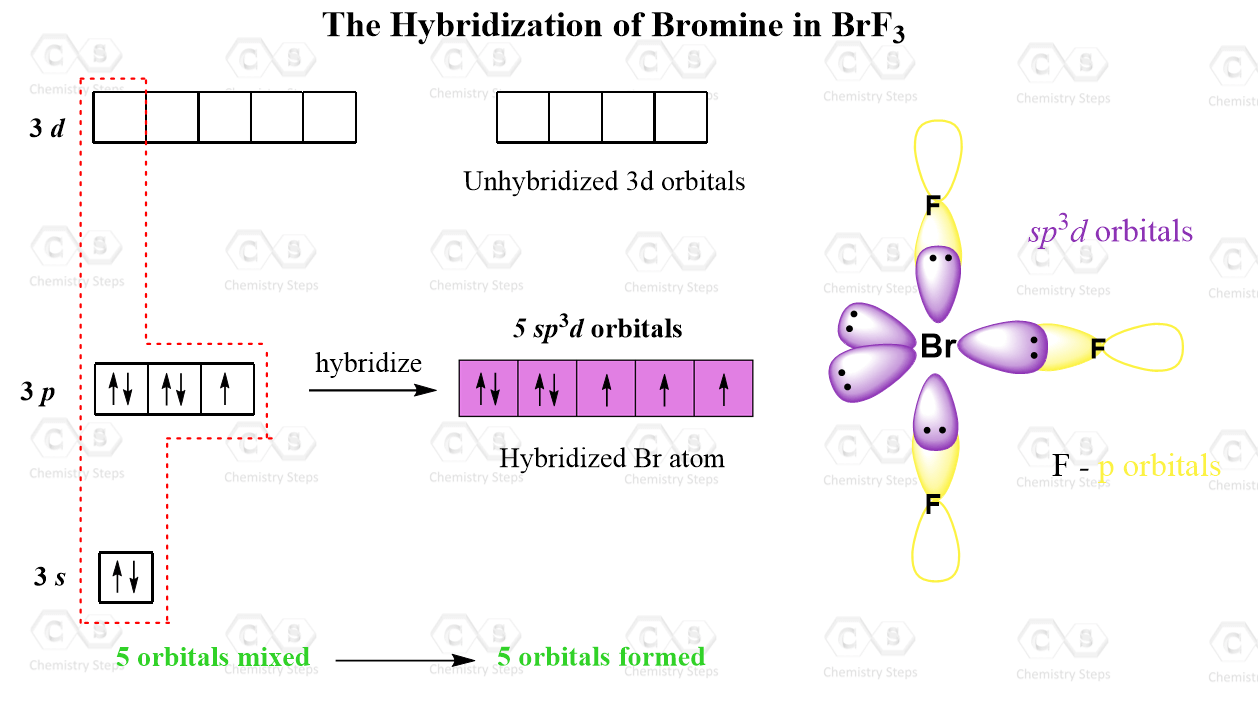
The hybridization and geometry of Br{F}_{3} molecules are:{ sp }^{ 3 }{ d }^{ 2 } and tetragonal{ sp }^{ 3 }d and T-shaped{ sp }^{ 3 }d and bentnone of these

SOLVED: Question 9 (10 points) What is the hybridization of the central atom in AsBr32- (OR AsBr3 2-)? sp sp² sp³ sp³d sp³d² Question 10 (10 points) What is the hybridization of

SOLVED: Of the following, the central atom is sp3d2 hybridized only in BeF2, AsCl3, PH3, Br3, O, and XeF4.