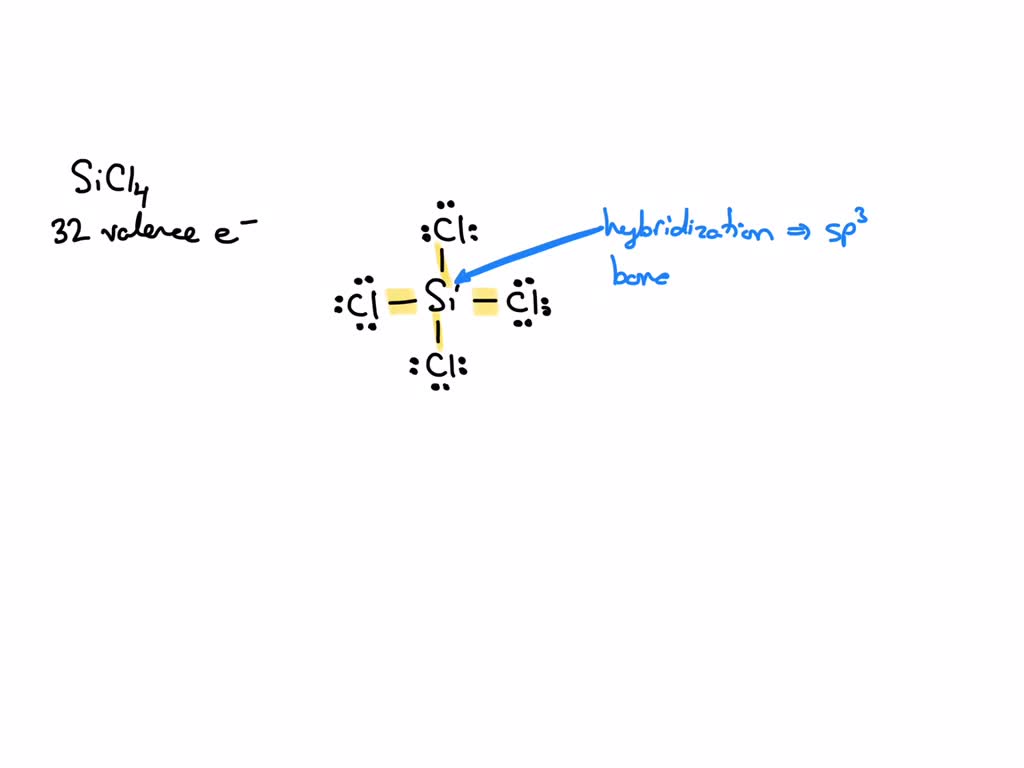
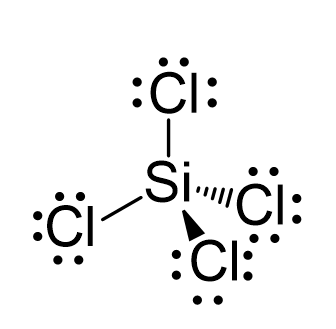
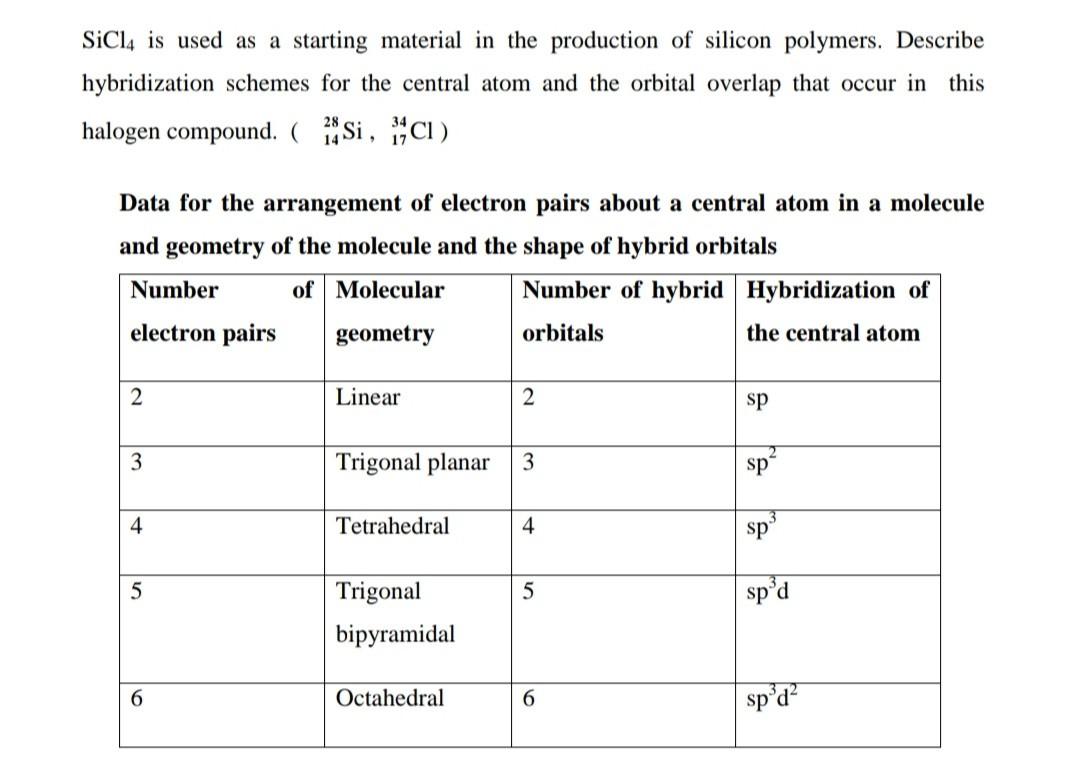
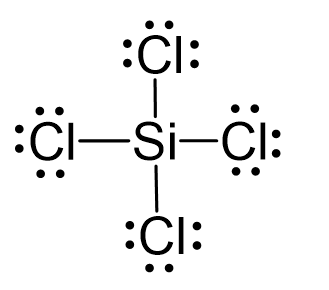
SOLVED: 4a) Using Valence Bond Theory, show the hybridization and bonding scheme for silicon tetrachloride (SiCl4): (a) write the atomic orbital diagram for the central atom, (b) circle the atomic orbitals that
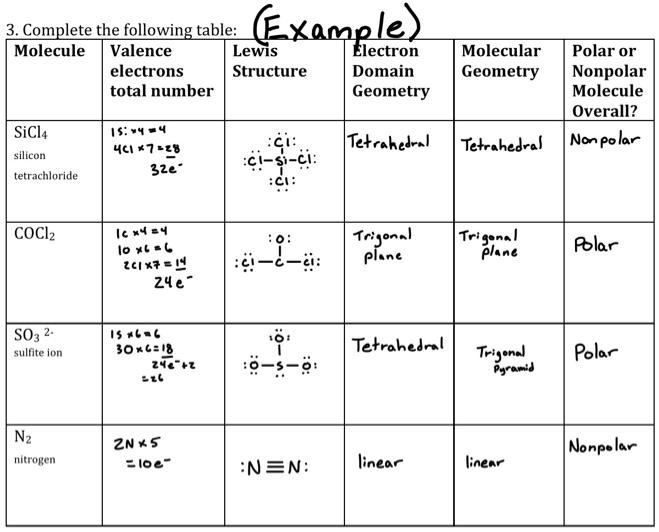
SOLVED: Complete the following table: Molecule Valence Lewis Electrons Structure Domain Total Number Geometry Molecular Geometry Polar or Nonpolar Molecule Overall? Nonpolar SiCl4 Silicon Tetrachloride 4 7-2-4 3-2-4 Tetrahedral Tetrahedral Nonpolar COCl2
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than
In case of hybridization in chemical bonding is only the central atom hybridized or atoms also except central atoms hybridize to form bonds? What is the exact concept of hybridization? - Quora
Hybridization is a phenomenon that takes place in an atom before chemical bonding. How is hybridization responsible for the observed structure of SiCl4? - Quora
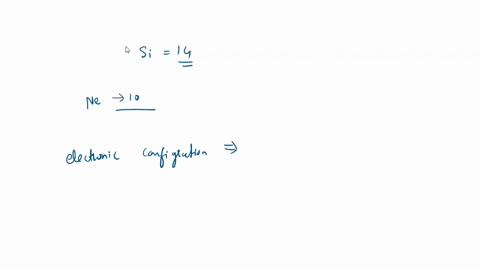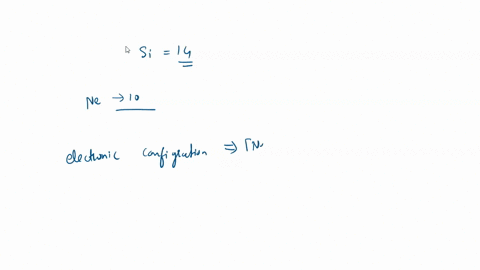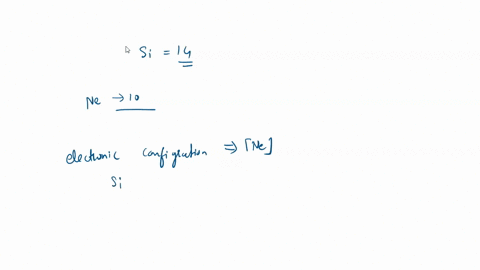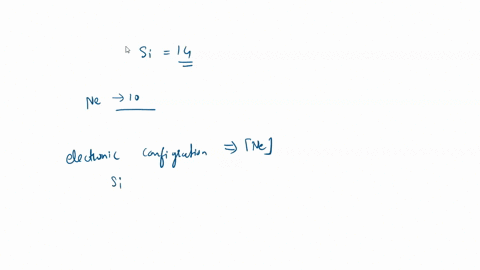
SOLVED: For the tetrahedral molecule, silicon tetrachloride, SiCl4, write the condensed electron configuration of a lone silicon atom, predict the hybridization in the molecule, and write an electron configuration for the hybridized orbitals of the central silicon ...